

Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in nitrosyl chloride: ClNO or ClON?.Determine the formal charge on each atom in each of the resonance structures: Draw all possible resonance structures for each of the compounds below.Thus, we calculate formal charge as follows: Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms.
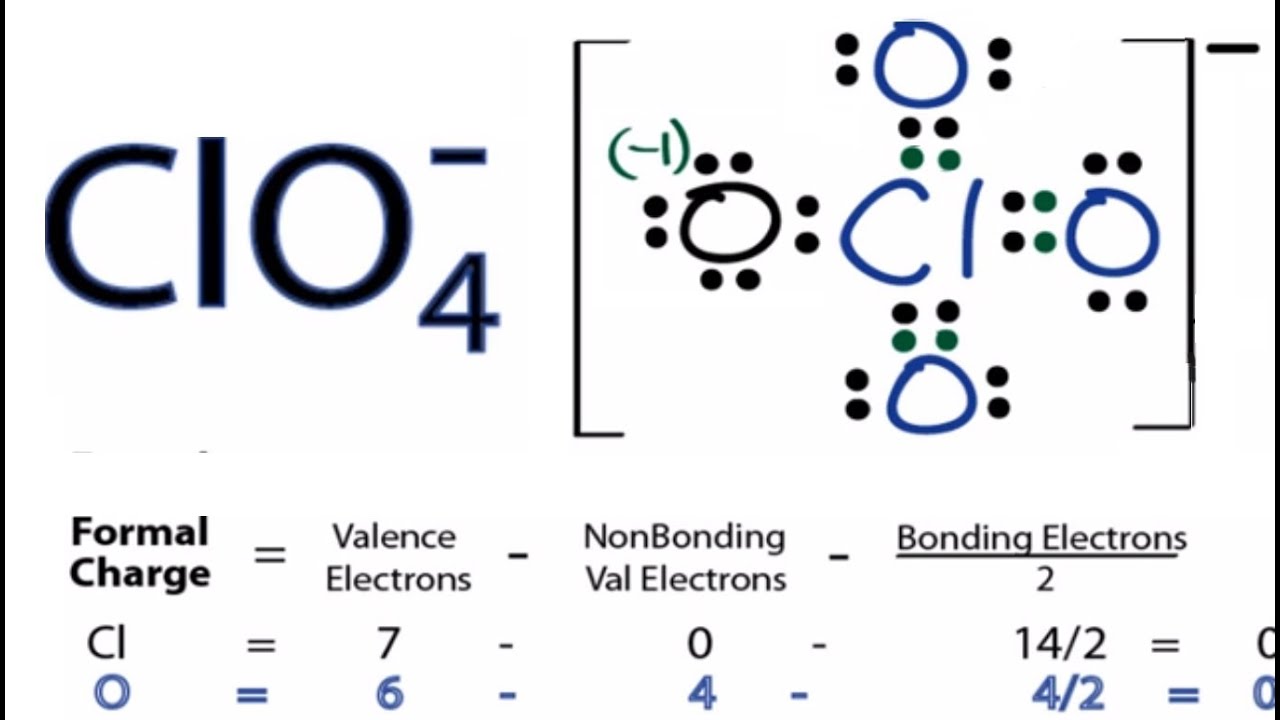
We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule.
Lewis structures calculating formal charge how to#
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions.

Use formal charges to identify the most reasonable Lewis structure for a given molecule.Compute formal charges for atoms in any Lewis structure.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
